How can nucleic acids predict disease?
Topics Featured
Liquid biopsy is a diagnostic technique where bodily fluids are examined for biomarkers that suggest disease. In recent years, liquid biopsy has been recognized as an easy and non-invasive means of preventive screening for various cancers, autoimmune and mitochondrial diseases, neurological disorders and prenatal diagnoses. The process is simple: blood is drawn, plasma is isolated and circulating cell-free nucleic acids (ccfNAs) are extracted from the plasma. These nucleic acids are then analyzed to identify possible mutations that may suggest propensity for diseases. Liquid biopsy can be a powerful tool for early diagnosis, as ccfNAs are often present in plasma long before tumor detection or other signs of disease.
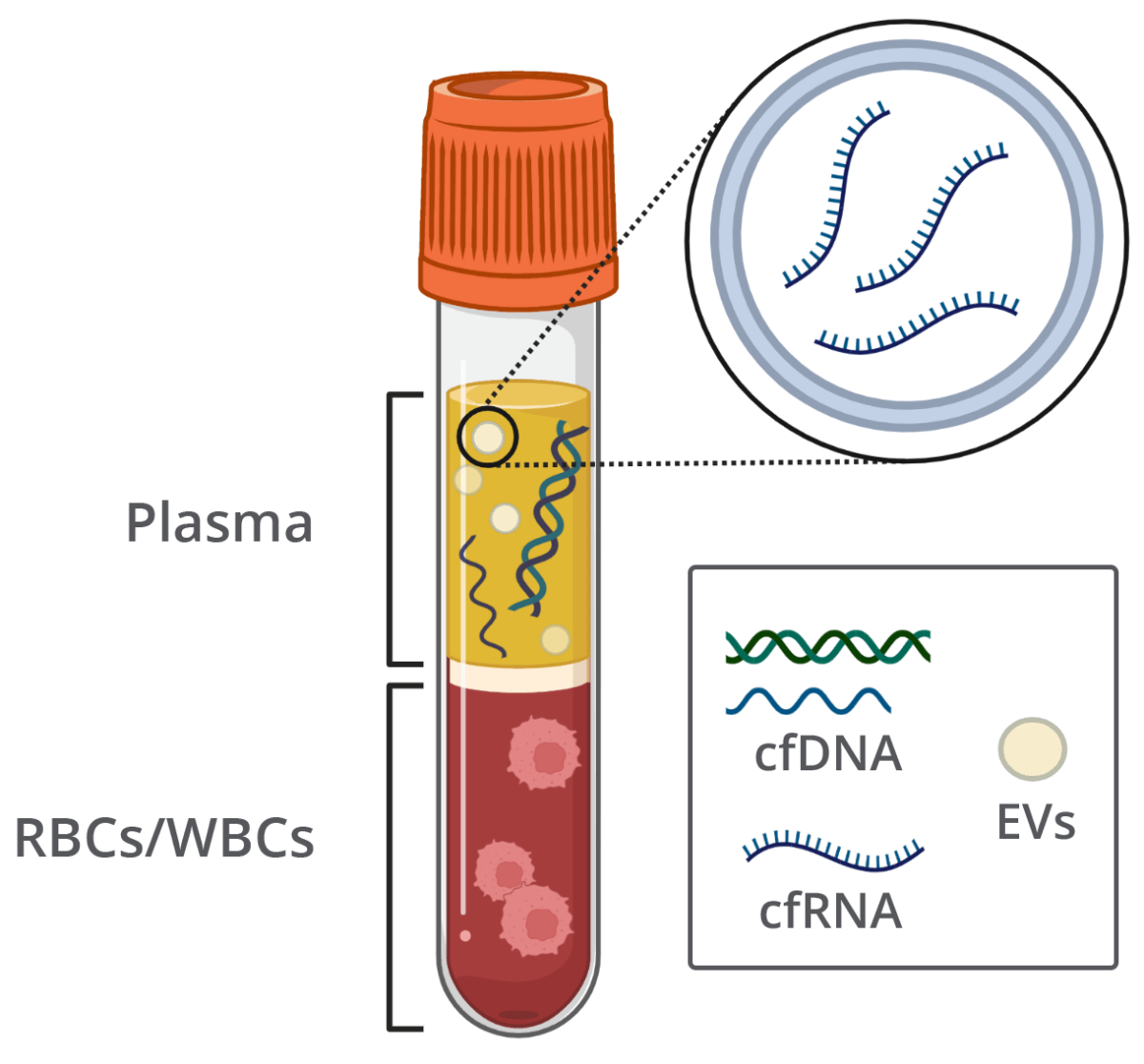
Several kinds of ccfNAs are found in circulating blood, including cell-free DNA (cfDNA) and cell-free RNA (cfRNA). cfDNA and cfRNA can be released into circulation via apoptosis (cell death), active secretion or necrosis (tissue death). In healthy individuals, ccfNAs originate in lymphoid and myeloid tissue. However, in instances of disease, ccfNAs can be released from the affected tissue or area into the bloodstream in abnormal levels. These levels can be an important indication of the condition causing ccfNA release. Studies have associated increased cfRNA levels with autoimmune diseases, such as lupus and rheumatoid arthritis, and increased cfDNA levels with certain pregnancy-related diseases.
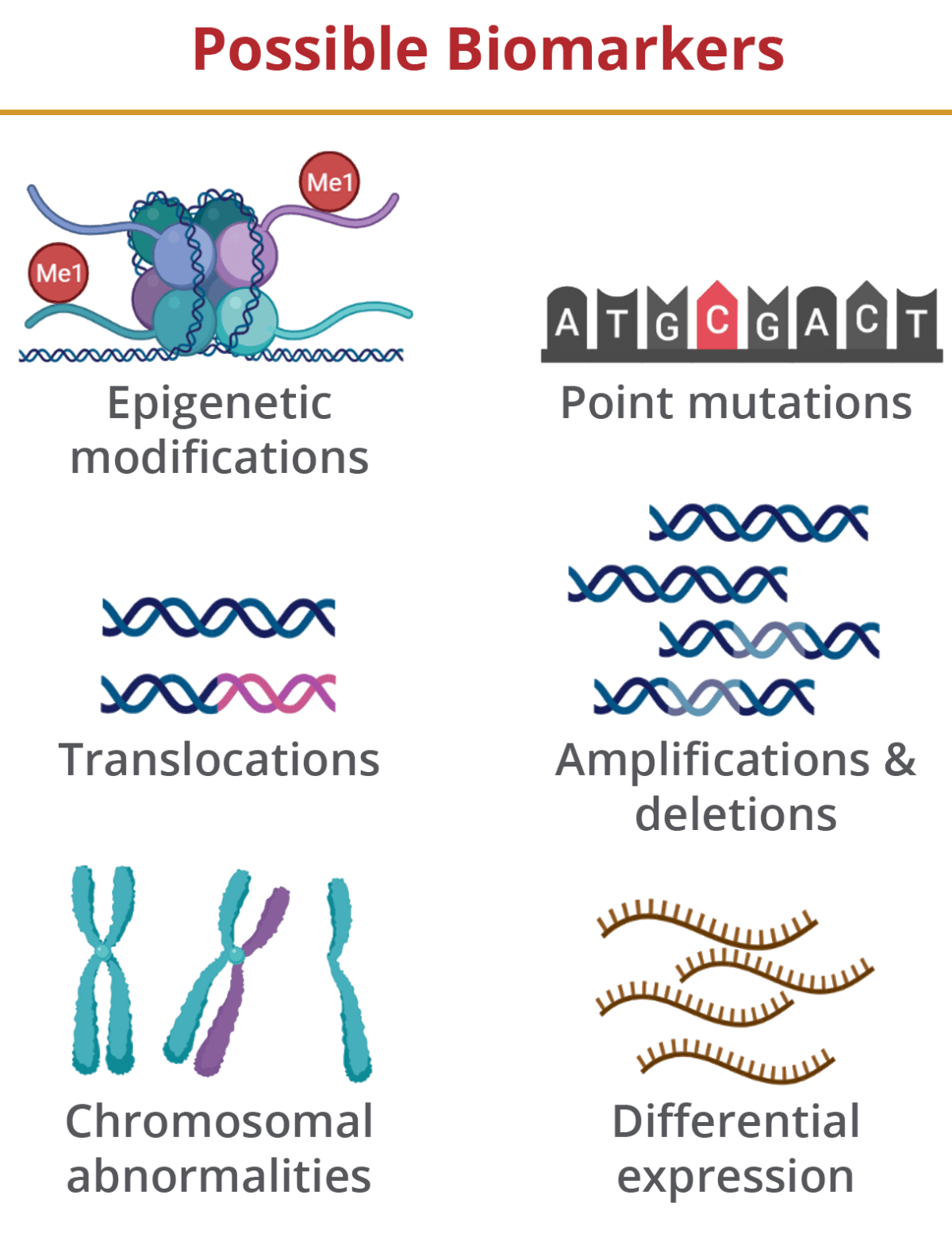
Most often, ccfNAs isolated from liquid biopsy samples are sequenced to identify mutations and modifications that are associated with particular conditions. Several cancers, including colorectal, breast and ovarian cancers, have been linked to mutations within certain genes that can be readily identified in cfDNA. Modifications to cfDNA have also been attributed to diseases such as preeclampsia and can indicate traumatic brain injury. Mutated cfRNA transcripts can act as biomarkers for neurological disorders, such as Alzheimer’s and Parkinson’s disease, and many pregnancy-related diseases, including preeclampsia and gestational diabetes. Given its ability to identify critical biomarkers for disease without the need for invasive measures, liquid biopsy has enormous potential for diagnostic medicine.
It is important to note that effective use of liquid biopsy as a prognostic measure depends on an accurate analysis of cfDNA, cfRNA and extracellular vesicle (EV) levels. However, blood analysis is often complicated by sample degradation during storage and shipping. Over time, white and red blood cells break down, contaminating the circulating nucleic acid population with genomic DNA, EVs and EV-associated cfRNA. If this plasma is then used for liquid biopsy, the analysis may not be reflective of the sample at draw time, and thus, may lead to incorrect diagnoses.
To address this issue, we developed Nucleic Acid BCT™, which stabilizes draw-time concentration of cfDNA, EVs and cfRNA during shipping and storage.
Nucleic Acid BCT is ideal for labs that want to:
- Reduce hemolysis in their samples
- Maximize plasma yield
- Maintain draw-time concentrations of cfDNA, EVs and cfRNA for up to 7 days of room temperature storage
- Develop new liquid biopsy assays

For Research Use Only. Not for use in diagnostic procedures.
Read more about how Nucleic Acid BCT maintains draw-time concentration of cfDNA, EVs and cfRNA here:
Liquid Biopsy and ccfNA overview
Rahat B, Ali T, Sapehia D, Mahajan A, Kaur J. Circulating Cell-Free Nucleic Acids as Epigenetic Biomarkers in Precision Medicine. Front Genet [Internet]. 2020;11. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2020.00844
Pös O, Biró O, Szemes T, Nagy B. Circulating cell-free nucleic acids: characteristics and applications. Eur J Hum Genet. 2018 Jul 1;26(7):937–45.
Epigenetic markers and disease
Zeng C, Stroup EK, Zhang Z, Chiu BCH, Zhang W. Towards precision medicine: advances in 5-hydroxymethylcytosine cancer biomarker discovery in liquid biopsy. Cancer Commun. 2019 Dec 1;39(1):12.
Origin of ccfNAs
Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell-free DNA Comprises an In Vivo Nucleosome Footprint that Informs Its Tissues-Of-Origin. Cell. 2016 Jan 14;164(1):57–68.
Swarup V, Rajeswari MR. Circulating (cell-free) nucleic acids – A promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007 Mar 6;581(5):795–9.
Devonshire AS, Whale AS, Gutteridge A, Jones G, Cowen S, Foy CA, et al. Towards standardization of cell-free DNA measurement in plasma: controls for extraction efficiency, fragment size bias and quantification. Anal Bioanal Chem. 2014 Oct 1;406(26):6499–512.
Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA Expression in Human Peripheral Blood Microvesicles. PLOS ONE. 2008 Nov 11;3(11):e3694.
Noferesti SS, Sohel MdMH, Hoelker M, Salilew-Wondim D, Tholen E, Looft C, et al. Controlled ovarian hyperstimulation induced changes in the expression of circulatory miRNA in bovine follicular fluid and blood plasma. J Ovarian Res. 2015 Dec 9;8(1):81.
Autoimmune disorders associated with cfRNA biomarkers
Li H, Li K, Lai W, Li X, Wang H, Yang J, et al. Comprehensive circular RNA profiles in plasma reveals that circular RNAs can be used as novel biomarkers for systemic lupus erythematosus. Clin Chim Acta. 2018;480:17–25. SLE
Jin F, Hu H, Xu M, Zhan S, Wang Y, Zhang H, et al. Serum microRNA profiles serve as novel biomarkers for autoimmune diseases. Front Immunol. 2018;9:2381. SLE and RA
Zheng F, Yu X, Huang J, Dai Y. Circular RNA expression profiles of peripheral blood mononuclear cells in rheumatoid arthritis patients, based on microarray chip technology. Mol Med Rep. 2017;16(6):8029–36. RA
cfDNA as biomarkers for pregnancy-related diseases
Romão RM, Levi JE, Carvalho HB de, Francisco RPV, Amorim Filho AG de, Zugaib M. Use of cell-free fetal nucleic acids in maternal blood for prenatal diagnosis: the reality of this scenario in Brazil. Rev Assoc Médica Bras. 2012;58:615–9. Hyperemesis gravidarum/cfDNA and Placenta accrete (increased levels).
Chim SS, Tong YK, Chiu RW, Lau TK, Leung TN, Chan LY, et al. Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc Natl Acad Sci. 2005;102(41):14753–8. Preeclampsia (increased levels).
Tsui DW, Chan KA, Chim SS, Chan L wai, Leung T yeung, Lau T kin, et al. Quantitative aberrations of hypermethylated RASSF1A gene sequences in maternal plasma in pre‐eclampsia. Prenat Diagn Publ Affil Int Soc Prenat Diagn. 2007;27(13):1212–8. Preeclampsia (increased levels).
Rahat B, Hamid A, Ahmad Najar R, Bagga R, Kaur J. Epigenetic mechanisms regulate placental c-myc and hTERT in normal and pathological pregnancies; c-myc as a novel fetal DNA epigenetic marker for pre-eclampsia. MHR Basic Sci Reprod Med. 2014;20(10):1026–40. Preeclampsia (mutation).
Rahat B, Najar RA, Hamid A, Bagga R, Kaur J. The role of aberrant methylation of trophoblastic stem cell origin in the pathogenesis and diagnosis of placental disorders. Prenat Diagn. 2017;37(2):133–43. Preeclampsia (mutation).
cfRNA as biomarkers for pregnancy-related diseases
25. Ura B, Feriotto G, Monasta L, Bilel S, Zweyer M, Celeghini C. Potential role of circulating microRNAs as early markers of preeclampsia. Taiwan J Obstet Gynecol. 2014;53(2):232–4. Preeclampsia.
26. Wu Z, Lu H, Sheng J, Li L. Inductive microRNA-21 impairs anti-mycobacterial responses by targeting IL-12 and Bcl-2. FEBS Lett. 2012;586(16):2459–67. Preeclampsia.
27. Grissa O, Yessoufou A, Mrisak I, Hichami A, Amoussou-Guenou D, Grissa A, et al. Growth factor concentrations and their placental mRNA expression are modulated in gestational diabetes mellitus: possible interactions with macrosomia. BMC Pregnancy Childbirth. 2010;10(1):1–10. Gestational diabetes.
28. Barchitta M, Maugeri A, Quattrocchi A, Agrifoglio O, Agodi A. The role of miRNAs as biomarkers for pregnancy outcomes: a comprehensive review. Int J Genomics. 2017;2017. Gestational diabetes.
cfDNA as biomarkers for cancer
Tham C, Chew M, Soong R, Lim J, Ang M, Tang C, et al. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer. 2014;120(20):3131–41. Colorectal cancer.
Semaan A, van Ellen A, Meller S, Bergheim D, Branchi V, Lingohr P, et al. SEPT9 and SHOX2 DNA methylation status and its utility in the diagnosis of colonic adenomas and colorectal adenocarcinomas. Clin Epigenetics. 2016;8(1):1–10. Colorectal cancer.
Kloten V, Becker B, Winner K, Schrauder MG, Fasching PA, Anzeneder T, et al. Promoter hypermethylation of the tumor-suppressor genes ITIH5, DKK3, and RASSF1A as novel biomarkers for blood-based breast cancer screening. Breast Cancer Res. 2013;15:1–11. Breast cancer.
Cheuk IWY, Shin VY, Kwong A. Detection of methylated circulating DNA as noninvasive biomarkers for breast cancer diagnosis. J Breast Cancer. 2017;20(1):12–9. Breast cancer.
Vu TL, Nguyen TT, Vo LTT. Methylation profiles of BRCA1, RASSF1A and GSTP1 in vietnamese women with breast cancer. Asian Pac J Cancer Prev APJCP. 2018;19(7):1887. Breast cancer.
Giannopoulou L, Chebouti I, Pavlakis K, Kasimir-Bauer S, Lianidou ES. RASSF1A promoter methylation in high-grade serous ovarian cancer: A direct comparison study in primary tumors, adjacent morphologically tumor cell-free tissues and paired circulating tumor DNA. Oncotarget. 2017;8(13):21429. Ovarian cancer.
Lønning PE, Berge EO, Bjørnslett M, Minsaas L, Chrisanthar R, Høberg-Vetti H, et al. White blood cell BRCA1 promoter methylation status and ovarian cancer risk. Ann Intern Med. 2018;168(5):326–34. Ovarian cancer.
ccfNAs as biomarkers for neurological disorders
Lorente L. Biomarkers associated with the outcome of traumatic brain injury patients. Brain Sci. 2017;7(11):142. cfDNA and TBI.
Bhatnagar S, Chertkow H, Schipper HM, Yuan Z, Shetty V, Jenkins S, et al. Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front Mol Neurosci. 2014;7:2. cfRNA and Alzheimer’s disease.
Jin XF, Wu N, Wang L, Li J. Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol Neurobiol. 2013;33:601–13. cfRNA and Alzheimer’s disease/cfRNA and Parkinson’s disease.
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–4. cfRNA and Alzheimer’s disease/cfRNA and Parkinson’s disease.

The Benefits of Glass BCTs