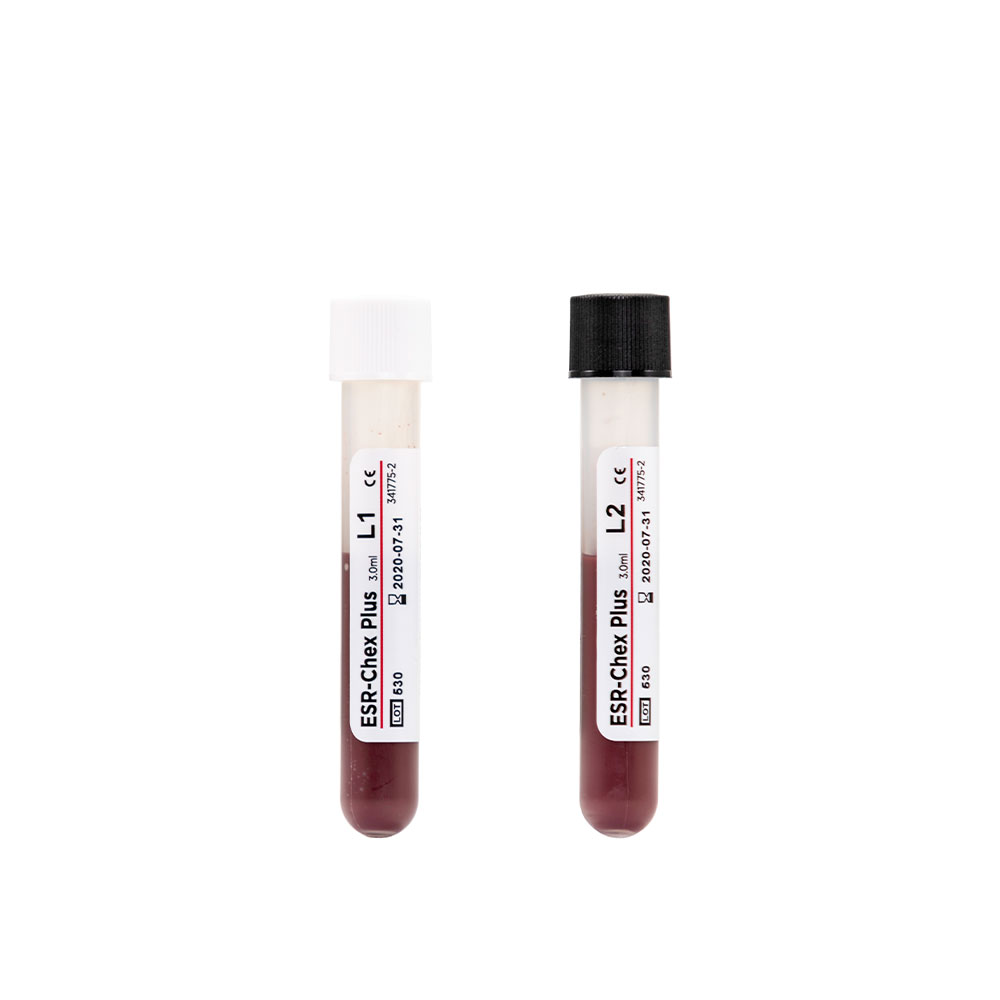
ESR-Chex®
ESR-Chex is a whole blood sed-rate control for automated and manual ESR testing methods. It is available in two levels and assayed for the most frequently used sedimentation rate tubes in conjunction with the Classical Westergren, Modified Westergren and Wintrobe methods.

Product Description
ESR-Chex is an assayed hematology control for evaluating the accuracy and precision of automated and manual ESR testing methods. This control is manufactured from human red blood cells and can be used in the same manner as a patient sample. ESR-Chex reacts to physical factors such as benchtop vibration, temperature and tube angle, highlighting possible variables that may affect the accuracy of patient results…

Features
- Ready-to-use whole blood control that resembles patient sample
- Available in two levels: normal and abnormal
- Assayed for both manual and automated sed-rate testing methods
- 95-day open-vial stability; 365-day closed-vial stability
Benefits
- Alerts the technologist to variables that may affect the accuracy of patient results
- Long shelf life reduces the number of shipments and associated costs
- Access to STATS®, our free interlaboratory quality control program for peer group data comparison
Ordering Information
| Description | Catalog Number |
|---|---|
| 4 x 9.0 mL (Level 1 & 2) | 214104 |
| 8 x 9.0 mL (Level 1 & 2) | 214108 |
| 12 x 9.0 mL (Level 1 & 2) | 214112 |
Resources
Instructions (IFU)
Product Information
Assays
Certificates
Looking for an older document? Click here for archived assays and certificates.
Sed-Rate Products
We can provide your laboratory with a comprehensive erythrocyte sedimentation rate (ESR) line that includes accurate, reliable and user-friendly analyzers, controls and manual methods for ESR testing.

The latest from the blog


CLIA Announces New 2024 Updates