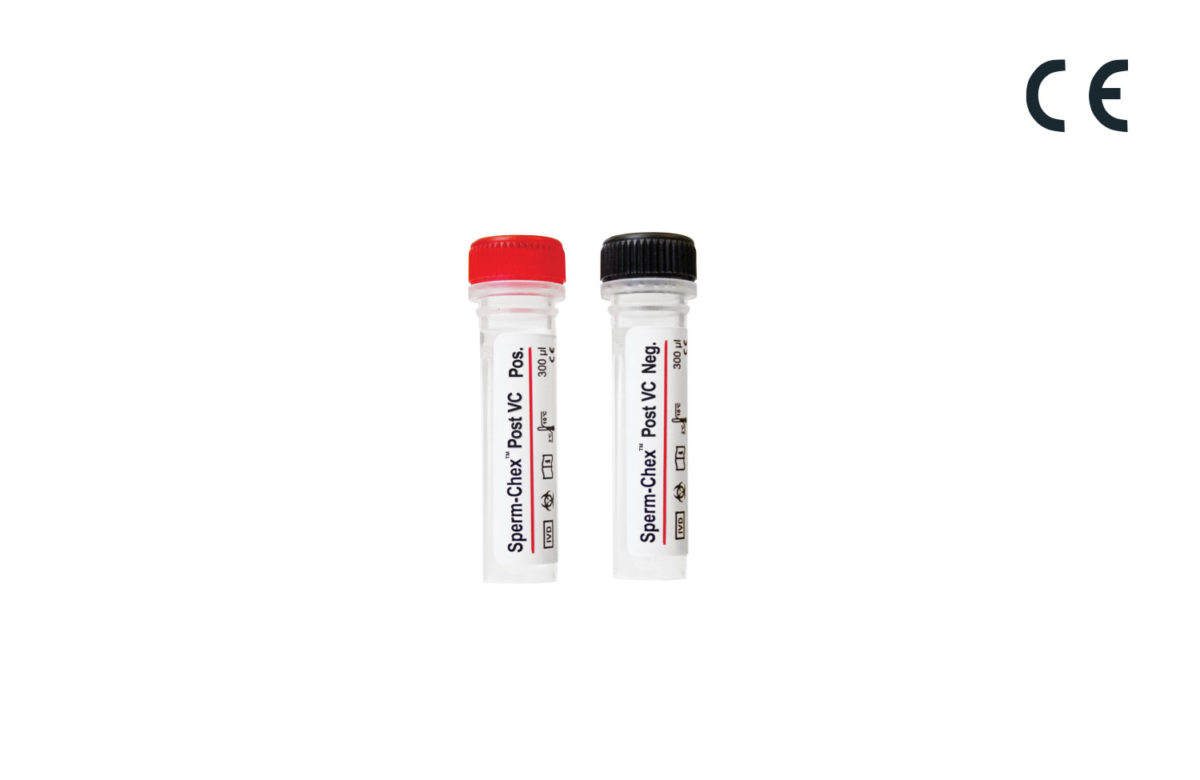
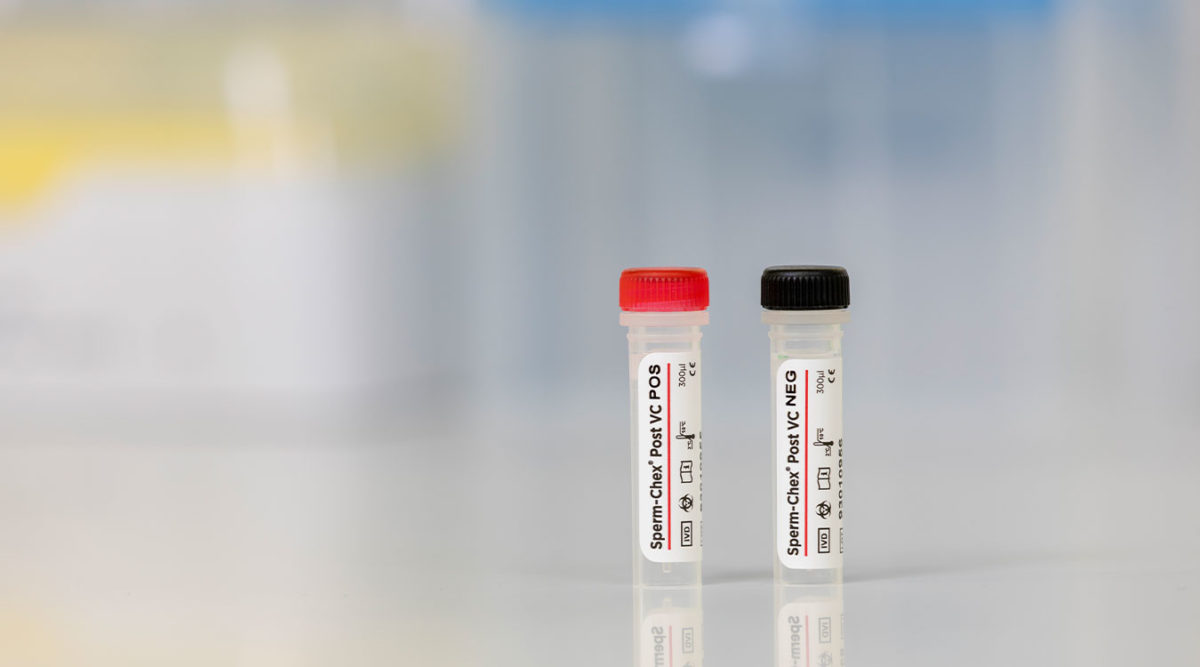
Sperm-Chex® Post VC
Sperm-Chex Post VC is a post-vasectomy sperm count control that validates the manual post-vasectomy sperm count procedure. Comprised of real stabilized sperm cells, Sperm-Chex Post VC can be handled like a patient sample and contains positive and negative sperm count samples to verify sperm quantification methods.
Product Description
Sperm-Chex Post VC is a positive/negative control that can be used to validate your laboratory’s manual post-vasectomy sperm counting procedure. This control contains stabilized sperm cells instead of latex beads to ensure patient sample-like chamber loading and optical characteristics. Sperm-Chex Post VC is assayed for use with hemacytometers and other commercially available manual counting chambers. It is available in a kit containing two 300 µL vials, one positive and one negative sperm count sample.
Features
- Provides a positive and negative sperm count sample
- Compatible with hemacytometers and other counting chambers
- 42-day open-vial stability; 12-month closed-vial stability
Benefits
- Helps clinical laboratories meet quality requirements for vasectomy sperm counts
- Performs the same as a patient sample for chamber loading
- Simulates the sperm cell concentration that lab technologists encounter in patient samples
- Long shelf life reduces the number of shipments and associated costs
Ordering Information
| Description | Item Number |
|---|---|
| 2 x 300 µL Positive, Negative | 230200 |
Order Sperm-Chex Post VC today
New customers
Existing customers
International customers find a distributor
Resources
Instructions (IFU)
Assays
Certificates
Product Information
Looking for an older document? Click here for archived assays and certificates.
Body Fluid & Urinalysis Products
With our line of manual and automated body fluid and sperm count controls, you can be certain in your body fluid testing methods and results.
The latest from the blog

Lab Horror Stories: The Tale of the Phantom Fluorescence

CLIA Announces New 2024 Updates