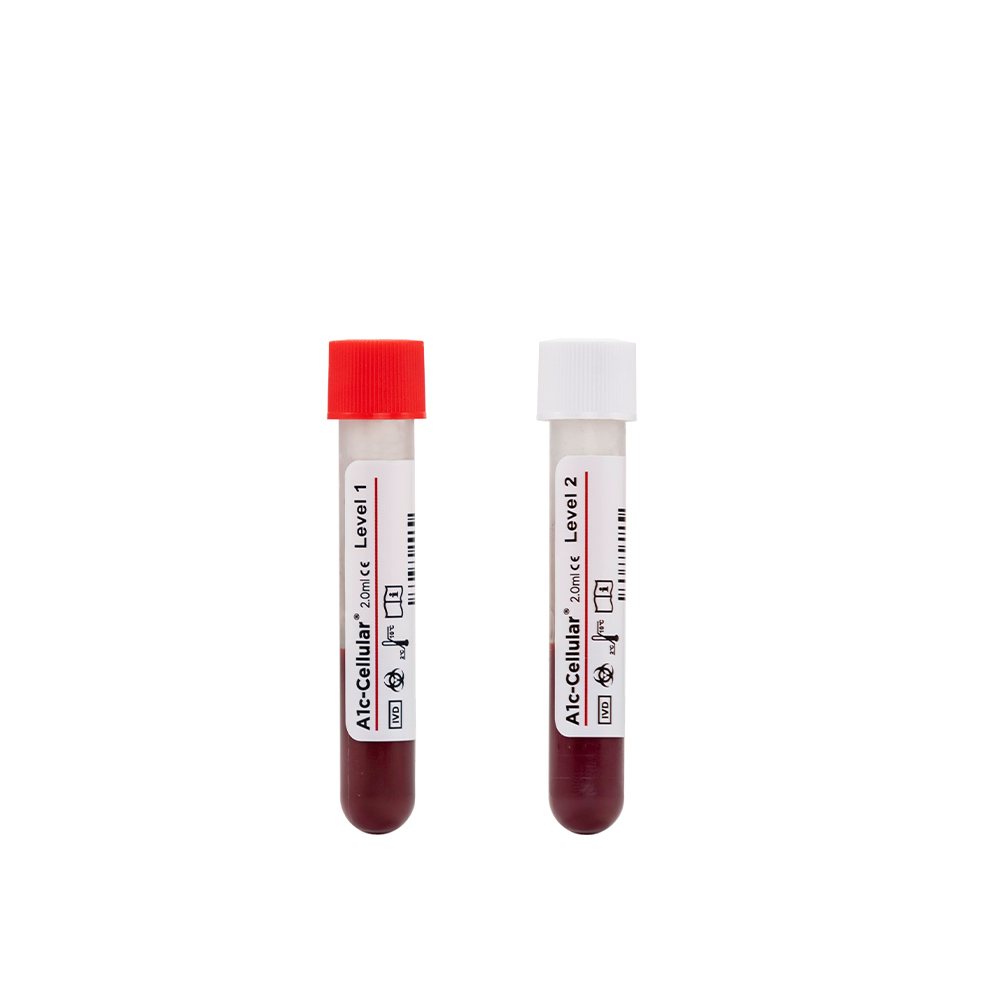
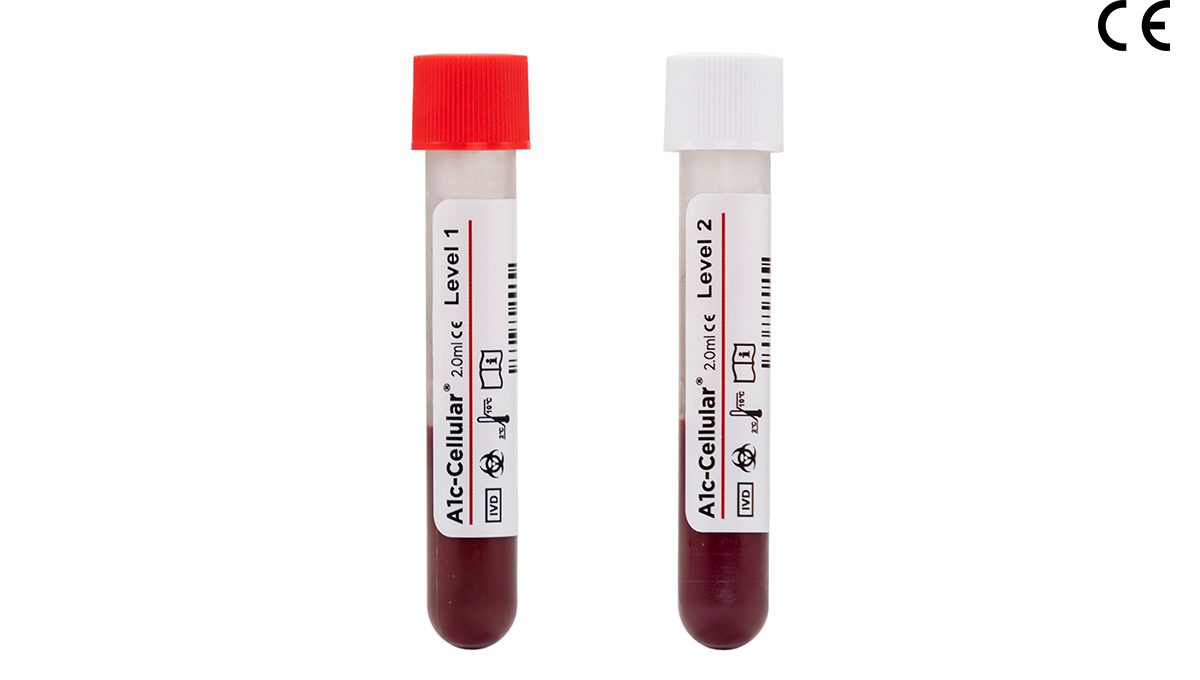
A1c-Cellular®
With A1c-Cellular, you can evaluate the entire HbA1c analysis process, from instrument to reagents, to ensure accurate patient results.
Our HbA1c product line provides you with controls that contain intact red blood cells to confirm the precision and accuracy of your laboratory’s instruments by challenging the entire HbA1c procedure, including erythrocyte lysis.
Streck customers also have access to STATS®, our free interlaboratory quality control program for peer group data comparison.
Product Description
A1c-Cellular is a whole blood control that tests the entire HbA1c procedure including red blood cell lysis, a step that is not evaluated by competitor controls. Designed to resemble a patient sample, A1c-Cellular does not need to be reconstituted prior to use, reducing the potential for human error. This control is packaged in convenient vials with pierceable caps for autosampling and is available in two clinically significant levels…
Features
- Available in two clinically significant levels
- Appropriate for immunoassay and ionic exchange HPLC methodologies
- Assayed on the top HbA1c analyzers
- 30-day open-vial stability; 190-day closed-vial stability
Benefits
- Tests the entire process including the lysis step
- Does not need to be reconstituted, reducing the potential for human error
- Access to STATS®, our free interlaboratory quality control program for peer group data comparison
Trust your entire HbA1c process to A1c-Cellular, the only control that includes the critical step of lysing the RBCs.
Ordering Information
| Description | Item Number |
|---|---|
| 2 x 2.0 mL (Level 1 & 2) | 211130 |
| 6 x 2.0 mL (Level 1 & 2) | 211124 |
Citations
Resources
View this product’s EU IVDR Declaration of Conformity
Instructions (IFU)
Assays
Certificates
Product Information
Looking for an older document? Click here for archived assays and certificates.
HbA1c Products
Streck’s HbA1c product line includes a control and linearity with intact red blood cells to challenge the entire HbA1c procedure, including the lysing of the red blood cells. Daily use of these whole blood controls provides quality control data for confirming the precision and accuracy of your laboratory’s instruments. Laboratories should validate test methods at least every six months or following changes in lots of critical reagents or system components.
The latest from the blog

Lab Horror Stories: The Tale of the Phantom Fluorescence

CLIA Announces New 2024 Updates